Which of the Following Guarantees a Reaction Will Be Spontaneous
Therefore with the help of the above relation spontaneity of a reaction can be easily predicted. An increase in free energy.
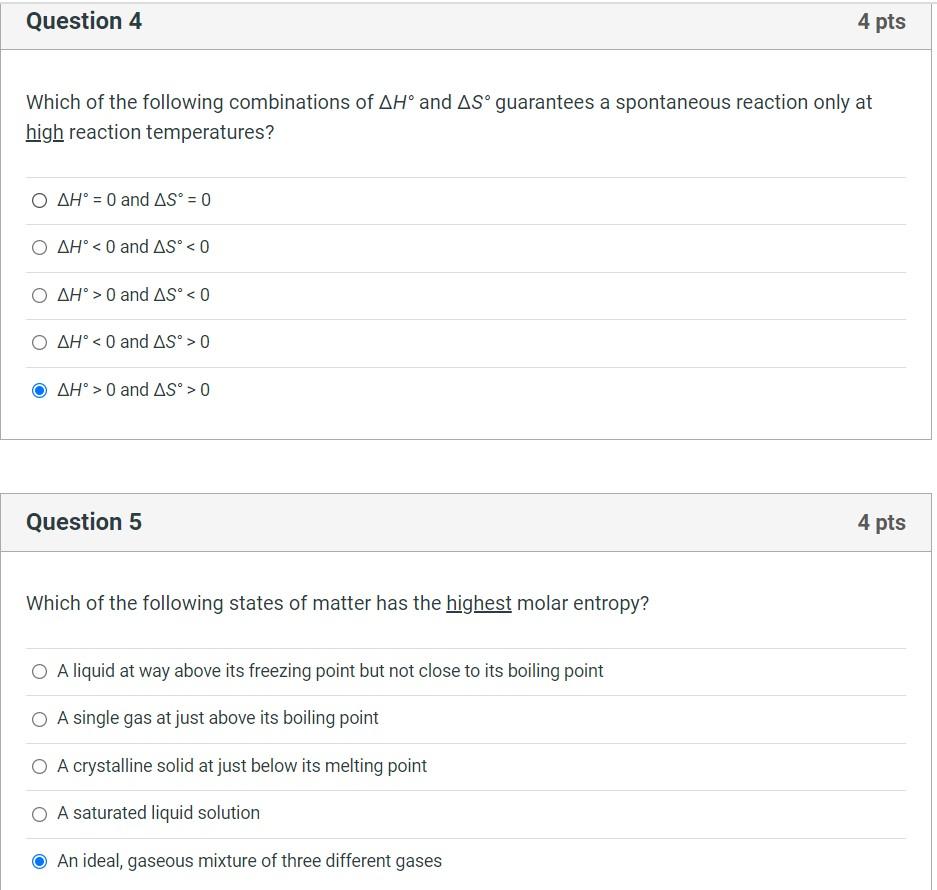
Solved Question 4 4 Pts Which Of The Following Combinations Chegg Com
Therefore ΔH should be negative and ΔS should be positive.
. Spontaneous at high temperatures where exothermicity is relatively unimportant ΔS negative ΔH negative. The standard enthalpy change for a reaction is H n H products m Ho reactants f f o Spontaneous Processes and Entropy A spontaneous process is a physical or chemical change that occurs by itself. ΔS positive ΔH positive.
Δ H 0 and Δ S 0. An increase in enthalpy. K2 T2 A2 a Organic Food Waste Decomposes.
C Honey Bees Perform A Hydrolysis Reaction To Convert Sucrose Into A Mixture. Thus it can be inferred that any process is spontaneous if the change in Gibbs energy of the system is less than zero or else the process is not spontaneous. ΔGΔH-TΔS for a reaction to be spontaneous ΔG should be negative.
Group of answer choices. There is no net change. A spontaneous reaction may involve an increase or decrease in enthalpy it may involve an increase or decrease in entropy but it will always involve a decrease in free energy that is a negative ΔG.
Which of the following combinations of Δ H and Δ S always guarantees a spontaneous reaction G 0 according to Δ G Δ H T Δ S. If Δ G is negative the reaction can be said to be spontaneous. Contrastingly if ΔH is positive and ΔS is negative the reaction is nonspontaneous at all temperatures as written.
Group of answer choices ΔH. A positive value for ΔG Gibbs free energy will guarantee a nonspontaneous reaction. The amount of free energy after the reaction is less than before the reaction.
Where n is the number of electrons involved F is the Faradays constant 96485 Cmol. A rock at the top of a hill rolls down. Which of the following must be true for a spontaneous exothermic process.
Using the Gibbs equation it can be said that. State whether or not the reaction is spontaneous. Δ H 0 and Δ S 0.
C The reaction will never reach equilibrium. An increase in entropy. The amount of disorder after the reaction is less than before the reaction.
Up to 10 cash back Correct answer. Δ H 0 and Δ S 0. It is spontaneous because the system undergoes _____ a.
0 and ΔS. If the reaction is not spontaneous would. If is negative the reaction is spontaneous it proceeds in the forward direction.
When ΔH is positive and ΔS is negative. A decrease in enthalpy. In order to determine whether a chemical reaction is spontaneous one has to consider both entropy and the enthalpy of the reaction in the form of Gibbs free energy.
If Δ S has a positive value T Δ S will be negative in nature because of a negative sign in front. With the results we can see that the reaction that would be most spontaneous is reaction b as the Gibbs free energy is the most. 0 and ΔS.
T is the reaction temperature and T 0. It can take place only when both terms in RHS is negative. Spontaneous at all temperatures.
As we do not know the number of electrons per reaction we will assume the same number for every reaction for the sake of the comparison. The standard free-energy of reaction is the free-energy change for a reaction when it. Process not spontaneous at any temperature.
A The reaction is spontaneous only above 225 K. Heat flows from a hot object to a cold one. When Gibbs Free Energy takes a.
For Each Of The Following State Whether The Event Or Process Is Spontaneous Or Non- Spontaneous And Under What Conditions You Would Expect It To Occur. 0 ΔH 0 and ΔS 0. The amount of free energy after the reaction is more than before the reaction.
Δ H 0 and Δ S 0. Which of the following statements is true of spontaneous reactions. A decrease in entropy.
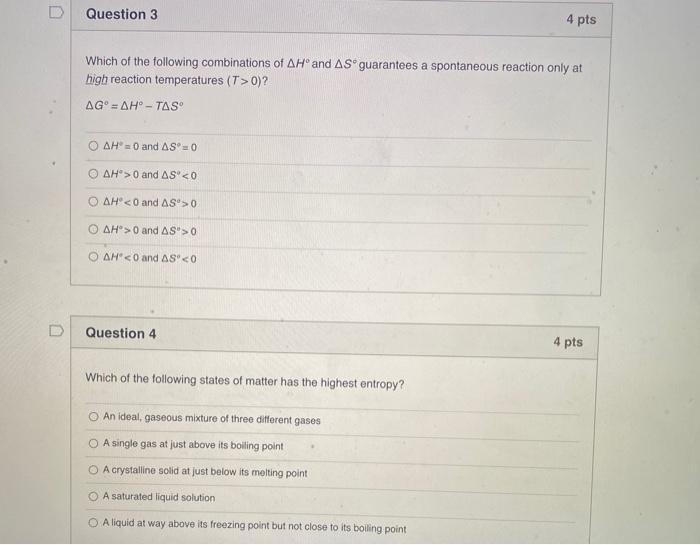
Which of the following combinations of ΔH and ΔS guarantees a spontaneous reaction only at high reaction temperatures. Spontaneity of a reaction can be given by the equation Δ G Δ H T Δ S. For each of the following reactions calculate ΔHrxn ΔSrxn ΔGrxn at 25 C.
If is positive the reaction is nonspontaneous it proceeds in the reverse direction. D The reaction is always at equilibrium. 0 and ΔS.
Spontaneous at low temperatures where exothermicity is dominant ΔS negative ΔH positive. B A Bacterial Cell Propels Itself Through Water Using A Flagellum. 0 and ΔS.
The dissolution of ammonium nitrate in water is a spontaneous endothermic process. When ΔH enthalpy is postive and ΔS entropy is negative the change in Gibbs free energy must be positive and therefore nonspontaneous. If ΔH is negative and ΔS is positive the reaction is spontaneous at all temperatures because the change in Gibbs free energy is always negative.
Which of the following would guarantee that a reaction would be spontaneous at constant T and P The Gibbs free energy of the system decreases this due to the fct hat reactions with a negative Gibbs free reaction releases energy and a spontaneous reaction is a reaction is a reaction that involves the release of energy by the reaction proceeding without any input of energy externally. Δ H 0 and Δ S 0. If 0 the system is at equilibrium.
B The reaction is spontaneous below 225 K. The reaction is not spontaneous would a change in temperature.

Solved Question 3 4 Pts Which Of The Following Combinations Chegg Com

Solved M1 Which Of The Following Conditions Guarantees That Chegg Com

Solved Which Of The Following Would Guarantee That A Chegg Com
No comments for "Which of the Following Guarantees a Reaction Will Be Spontaneous"
Post a Comment